expanded octet examples|Octet Rule : Tagatay Expanded octets are possible in Lewis Structures because the elements in Period Three and below have d orbitals that can take part in bonding. Two common . Pahindot Hingang Malalim Bago Isubo ang Utin. 2 days ago. Chix ni Bulog Magaling Sumubo ng Itlog. 4 days ago. Nakafighting Position Agad Ang Panabong ni Anton. 1 week ago. Ang Sabi Hihiram Lang ng Charger Pero Iba Ang Sinaksak ni Makmak. 1 week ago. Nang Bumulusok Ang Pantusok ni Bornok.Since 1995. Our goal is to provide our customers quality products at competitive pricing. We are committed to the needs of our customers and work with you to meet your goals. We want to be your one stop shop for .
expanded octet examples,For example, the Lewis structure of SF 6 can be redrawn with two ionic S-F bonds and four covalent S-F bonds as shown in Figure \(\PageIndex{1}\). In this picture the central S atom has six F atoms coordinated, but only shares four bonding pairs of .
An example of this would be if all of the outer atoms in a molecule were sitting in .
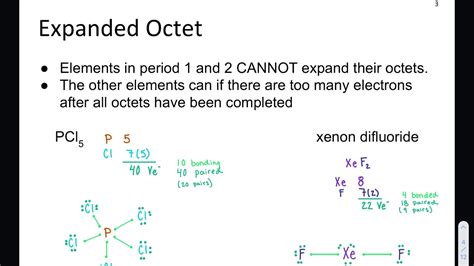
Expanded octets are possible in Lewis Structures because the elements in Period Three and below have d orbitals that can take part in bonding. Two common .
Expanded octets are commonly observed in compounds involving these elements, particularly in molecules with central atoms surrounded by a larger number of bonded .
In this video, we discuss the topic of expanded octets, which is an exception to the octet rule in which period 3 or higher elements can be surrounded by mor. An example of this would be if all of the outer atoms in a molecule were sitting in the nodal plane of a p-orbital. We do not have that case in this example, so now we need to dig a little deeper. Our next .Sulfur, phosphorus, silicon, and chlorine are common examples of elements that form an expanded octet. Phosphorus pentachloride (PCl 5) and sulfur hexafluoride (SF 6) are examples of molecules that deviate . Explanation and examples of how to write expanded octet Lewis Dot Structures. Watch more expanded octet examples here: .The octet of the central sulfur atom has been expanded to hold 10 electrons. Chlorine trifluoride, ClF 3. The total number of valence electrons is = Cl + 3F = 7 + (3 x 7) = 28. .Octet Rule In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements . Lewis Structures of Covalent Molecules that Contain Atoms with Expanded Valences. d orbitals can, however, impact the covalent interactions of non-metals and metalloids. Recall that electron dot . One important exception to the Octet Rule is that some elements can have Lewis Structures with expanded octets. Elements in Period Three and below on the Pe.Write Lewis structures for the following: (please note, none of the solutions are using the expanded octet rule or formal charges) H 2; HBr; PCl 3; SF 2; H 2 CCH 2; HNNH; H 2 CNH; NO – N 2; CO; CN – Answer a. Answer .

Exception 3: Expanded Valence Shells. Example 3: The \(ICl_4^-\) Ion. Solution; Practice Problems; Answers; . are treated as more favorable than Lewis structures that follow the octet rule is when the formal charges in the expanded octet structure are smaller than in a structure that adheres to the octet rule, or when there are . Above: Three examples of Lewis structures for which electrons surrounding the central atom must be expanded beyond an octet. Xenon tetrafluoride (XeF 4), sulfur pentachloride (SF 5), and phosphorous hexafluoride (SF 6). Incomplete octets. In some cases, when the procedure for drawing a Lewis structure is followed, the result is a .An example of this would be Nitrogen (II) Oxide (NO, refer to figure one). Nitrogen has 5 valence electrons while Oxygen has 6. . One of the situations where expanded octet structures are treated as more favorable than Lewis structures that follow the octet rule is when the formal charges in the expanded octet structure are smaller than in a .expanded octet examples In this video, we discuss the topic of expanded octets, which is an exception to the octet rule in which period 3 or higher elements can be surrounded by mor.Example \(\PageIndex{2}\): Octet Rule Violations. Xenon is a noble gas, but it forms a number of stable compounds. We examined XeF 4 earlier. What are the Lewis structures of XeF 2 and XeF 6? Solution. We can draw the Lewis structure of any covalent molecule by following the six steps discussed earlier. In this case, we can condense the last . This video explains how elements in Period 3 and below can expand their octet to allow for bonding with other atoms and having more than 8 electrons in their.
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride (PCl 5), sulfur hexafluoride (SF 6), chlorine trifluoride (ClF 3), the chlorite .Expanded octet: Some molecules like SF 6, PCl 5, H 2 SO 4 have more than eight electrons around the central atom. SF 6; 12 electrons around sulfur PCl 5; 10 electrons around Phosphorus H 2 SO 4; 12 electrons . In organic chemistry the octet rule is closer to a law; do not make more than four bonds to carbon! However, once you start dealing with atoms in the third row of the periodic table, the octet rule becomes a bit .
These elements, often the nonmetallic elements in these periods, can exceed the octet rule to form molecules with more than four bonds resulting in an expanded octet. Examples in this category are .
Can atoms like Be and Al have form enough bonds to form a full octet. Yes, if another atom donates a lone pair (not just one electron) into the empty orbital, this can happen. We will . 2. Atoms with an expanded octet. To have an expanded octet (more than 8 electrons) you need more than 4 orbitals.

Other articles where expanded octet is discussed: chemical bonding: Hypervalence: .Lewis terms, hypervalence requires the expansion of the octet to 10, 12, and even in some cases 16 electrons. Hypervalent compounds are very common and in general are no less stable than compounds that conform to the octet rule.expanded octet examples Octet Rule Other articles where expanded octet is discussed: chemical bonding: Hypervalence: .Lewis terms, hypervalence requires the expansion of the octet to 10, 12, and even in some cases 16 electrons. Hypervalent compounds are very common and in general are no less stable than compounds that conform to the octet rule.
Examples from the p-block elements include SF 6, a substance used by the electric power industry to insulate high-voltage lines, . has a formal charge of 0. Thus by using an expanded octet, a +2 formal charge on S can be eliminated. Less . Here are a few examples of elements that do not strictly follow the octet rule: Hydrogen: It only accommodates 2 electrons in its valence shell (to achieve the configuration of helium), so it does not follow the octet rule.; Helium: Similarly, helium’s valence shell is complete with just two electrons.; Lithium and Beryllium: In the second . Examples of Elements with Expanded Octet. Some elements can expand their octets, meaning they can accommodate more than eight electrons in their outer shell. This phenomenon occurs when atoms form molecules or compounds and allows these elements to achieve greater stability. One example of an element that exhibits .
expanded octet examples|Octet Rule
PH0 · The Expanded Octet
PH1 · Octet Rule
PH2 · Lewis Theory XII: Expanded Octets
PH3 · Lewis Structures for Compounds with Expanded Octets
PH4 · Hypervalent molecule
PH5 · Expansion of the Octet (HL)
PH6 · Expanded Octet
PH7 · 3.3C: Expanded Octets
PH8 · 2.7.4: Expanded Octets and Molecular Orbitals